Cytoskeletal organization of regulatory circuits in
skin development, wound healing, and cancer
Building barriers
To function as effective barriers, epithelial tissues must form stable structures that resist mechanical insult. Yet stability must be balanced with plasticity to support tissue remodeling in contexts such as development and wound healing. We study the molecular circuits controlling the critical stability/plasticity balance and how disruption of these signaling networks contributes to disease. In particular, we focus on the intermediate filament cytoskeleton, not for its canonical role as a mechanical scaffold, but as a signaling scaffold modulating cell function.
We use the epidermis as a model of the stability/plasticity balance required in all epithelial tissues. As the interface between the organism and the outside world, the skin is routinely subject to mechanical stress. The skin also possesses remarkable regenerative capacity, with the plasticity to repair defects ranging from minor abrasions to significant lacerations. Many skin diseases ultimately represent loss of stability/plasticity balance, including non-healing wounds (too much stability), keratinocyte carcinomas (too much plasticity), and many others.
Keratin intermediate filaments
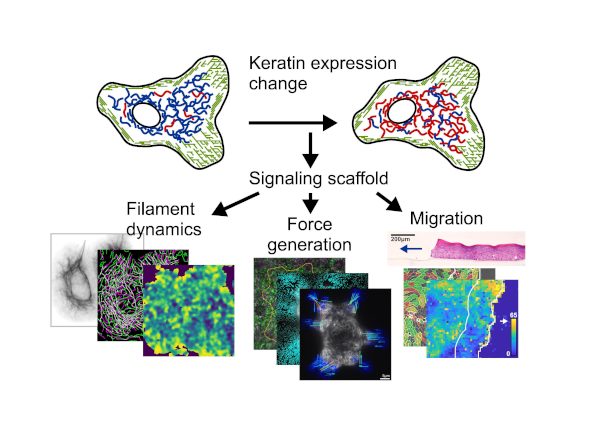
The keratin intermediate filament cytoskeleton, joined between adjacent cells through desmosomes, forms a strong mechanical scaffold ensuring epidermal stability. In contrast to the actin and microtubule cytoskeletons, intermediate filaments are remarkable diverse. Fifty-four different keratin isoforms are expressed in different combinations depending on tissue, anatomic site, and cell differentiation state. This diversity allows keratin filaments to function not only as mechanical scaffolds, but also as signaling scaffolds, with cellular circuits organized and tuned by the specific keratin isoforms expressed.

Our approach
Historically, identifying specific functions of individual keratin isoforms has been difficult, both due to multiple redundancy among isoforms and due to broad disruption of the keratin mechanical scaffold caused by depletion or mutation of individual keratins. As a result, while loss-of-function studies, including animal models and human inherited diseases, have underscored the importance of the keratin mechanical scaffold and the intricate pattern of keratin isoform expression, the reasons keratin isoforms are so intricately patterned have remained unclear.
We overcome these challenges using minimal-intervention and gain-of-function approaches to modulate the keratin isoform mixture while preserving overall keratin expression levels and the mechanical scaffold. Using skin organoid culture, genome engineering (CRISPR/Cas, endogenous tagging, tunable expression systems, etc.), live imaging, proteomics, and computational analysis, we seek to uncover the cellular circuits underlying complex epithelial tissue behaviors.
Areas of interest

Wound healing
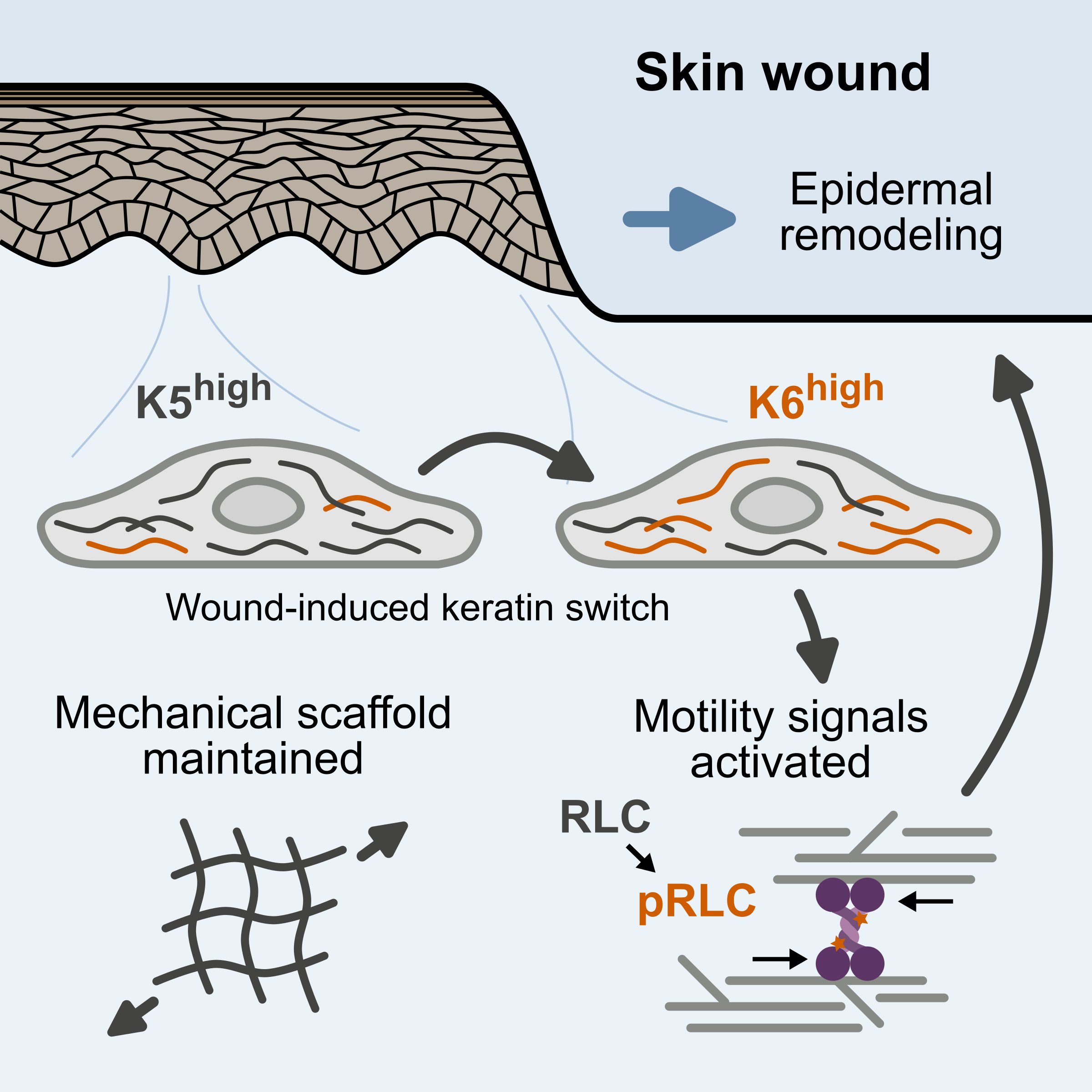
During skin wound healing, the keratin isoform mixture changes, with increased expression of five wound-associated keratin isoforms. We have found that this shift activates a signaling pathway leading to increased cellular force generation through actomyosin contractility. This mechanism provides a functional link between the cellular machinery ensuring mechanical stability and driving tissue remodeling in precisely the context where the stability/plasticity balance needs to be tuned. We are continuing to investigate the role of this pathway in wound healing and epidermal remodeling.
Epithelial to mesenchymal transition
Much to our surprise, we found that the mechanistic link between wound-associated keratins and actomyosin activation depends on a signaling complex anchored by vimentin, an intermediate filament protein typically associated with epithelial to mesenchymal transition (EMT), for example, during cancer metastasis. We are investigating the interplay between vimentin and keratins as the stability/plasticity balance shifts and the mechanistic relationships between vimentin signaling, EMT, and wound healing.
Epidermal differentiation and epithelial specialization
The keratin isoform mixture also changes during epidermal differentiation, and specific keratin isoforms are expressed at special anatomic sites such as the palms and soles. We are investigating how these differentiation-associated and site-specific keratins not only reflect cell state, but influence cell behavior.

Keratin related diseases
Keratin gene mutations cause a number of inherited skin diseases, including epidermolysis bullosa simplex, epidermolytic ichthyosis, pachyonychia congenita, and others. Identification of keratin mutations as the cause of these disorders was instrumental in our understanding of the importance of keratin intermediate filaments as mechanical scaffolds. We are investigating how these rare diseases may also inform our understanding of keratin filaments as signaling scaffolds, and how targeting keratin-anchored cellular circuits may provide novel therapies for these and other skin diseases.